New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events
The purpose of this article is to update the changes to the author’s protocols used to manage acute filler related vascular events from those previously published in this journal. For lack of a better term, this new protocol has been called the High Dose Pulsed Hyaluronidase protocol(HDPH) for vascular embolic events with hyaluronic acid (HA) fillers. The initial protocol used involved many different modalities of treatment. The current protocol is exceedingly simple and involves solely the use of hyaluronidase in repeated high doses. Despite the simplicity of the treatment, it has proven itself to be very successful over the past two years of clinical use.
There has been no partial or complete skin loss associated with this protocol since its implementation if the protocol was implemented within 2 days of the ischemic event onset. The protocol involves diagnosis and repeated administration of relatively high doses hyaluronidase (HYAL) into the ischemic tissue repeated hourly until resolution (as detected clinically through capillary refill, skin color, and absence of pain). The dosage of HYAL varies as the amount of ischemic tissue, consistent with the new underlying hypothesis that we must flood the occluded vessels with a sufficient concentration of HYAL for a sufficient period of time in order to dissolve the HA obstruction to the point where the products of hydrolysis can pass through the capillary beds.
Although vascular embolic events are rare, it is important to note that the face has higher risk and lower risk areas for filler treatment, but there are no “zero risk” areas with respect to filler treatments. Even with good anatomic knowledge and correct technique, there is still some nonzero risk of vascular embolic events (including highly skilled, experienced injectors). However, with careful low pressure, low volume injection technique, and adequate preparation for treatment of acute vascular events, the risk is quite manageable and the vast majority of adverse events are very treatable with an excellent prognosis, with a few exceptions. This new protocol offers excellent results, but requires further research to determine optimal parameters for various HA fillers.
Hyaluronidase Protocol:
ESSENTIAL CONCEPTS REGARDING RESOLUTION OF FILLER-RELATED VASCULAR ADVERSE EVENTS
This article summarizes a new approach to the management of acute accidental intravascular embolism with hyaluronic acid (HA) fillers. As with the former protocol previously published,1 avoidance of complications is still the best strategy (Table 1). The old protocol involved a daily single treatment with hyaluronidase (HYAL) of 450 to 600 iu, as well as other modalities of treatment such as nitropaste, hyperbaric oxygen etc. In contrast, the new hyaluronidase protocol involves HYAL dosing based on the volume of ischemic tissue, with hourly repeated dosing to maintain high concentrations of HYAL throughout the ischemic zone. Treatment is based on the clinical determination of the approximate surface area of ischemic tissue (as determined clinically by observation of skin color and capillary refill), with larger doses of HYAL used for larger areas of involvement. This report is based on the author’s clinical experience as a consulting physician with several dozen cases in the past two years using this new high dose hyaluronidase protocol, in comparison to clinical experience in over 50 cases with previous hyaluronidase protocols.
The author assumes that the pathology of filler related ischemia is due to arterial embolism of filler that typically occurs immediately at the time of filler treatment, and that the solution is to flood the tissues containing the obstructed vessels with HYAL in sufficient concentration for a sufficient length of time to relieve the HA obstruction. Rarely, the clinical history is that the patient was completely normal at the termination of treatment, and that signs of ischemia started at some time (typically hours) later. This phenomenon of “delayed onset” is discussed further below.
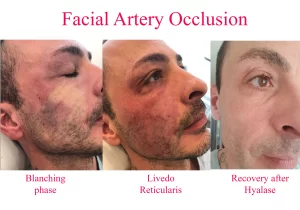
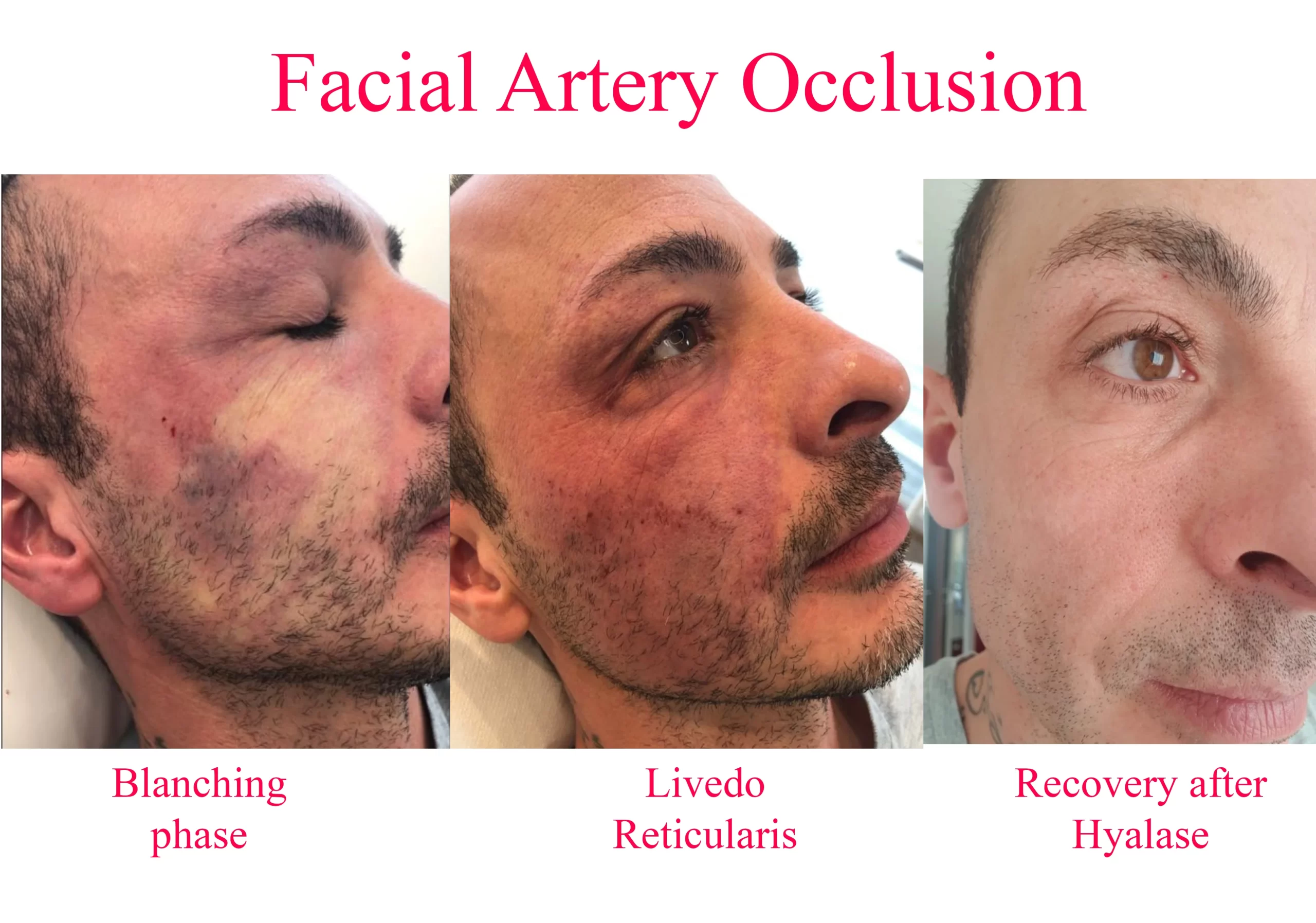
The natural history of vascular embolic events (ie, untreated) progresses from momentary blanching (which may only last a few seconds), through livedo reticularis (up to a few days), to blisters (day 3), crusting, necrosis, slough, and finally healing by secondary intention − a process that may take six weeks or more. If untreated, it takes approximately 3 days before blisters appear on the skin (this seems to be a relatively consistent finding), and frank necrosis may not be evident for several days thereafter (typically after day 6), but the signs of ischemia are generally present right from the very beginning if you look for them. With appropriate treatment with the new High Dose Pulsed Hyaluronidase protocol, we typically see complete reversal of all the signs of ischemia and complete return to normal.
Thus, instead of 6 weeks or more of slow healing by secondary intention, we see complete resolution with no signs of secondary problems within 3 days of the event, with patients typically suffering no more than a few bruises and so called “injection site reactions” (the normal sequelae of repeated needle injections). Diagnosis is completely clinical in nature. It involves examination of the skin, noting its color and in particular its capillary refill time. Typical cases of vascular obstruction may show some blanching, but this is often missed, since it is only momentary. A mottled skin appearance, termed livedo reticularis, is almost always apparent (except in cases of severe bruising).
Skin capillary refill test
Capillary refill time is noted to be very slow. Using the finger holes of an instrument (such as the non business end of a pair of suture scissors, for example) help to assess the capillary refill (Figure 1).(A) When determining the status of the skin’s capillary refill time, it is helpful to use and instrument to compress the skin. Patterns that are not typical in nature are more easily discernible. (B) The objective of this clinical test is to compare the refill time of the zone in question to normal skin either adjacent or on the contralateral side.
The first major breakthrough in the treatment of vascular adverse events (AE), was the administration of HYAL (the author believes that the ancillary treatments such as nitropaste etc. probably did not contribute very much to the final outcome). With the old hyaluronidase protocol, the author occasionally saw some blistering, crusting, and eventually some mild scarring, mild to moderate dyschromia (particularly in patients of color) despite treatment with HYAL. These occasional problems seemed particularly worse if more regions of the face had been originally involved in the vascular AE (eg, ischemia of the ipsilateral upper and lower lip, nose, and glabella) compared to single site (lip ischemia alone).
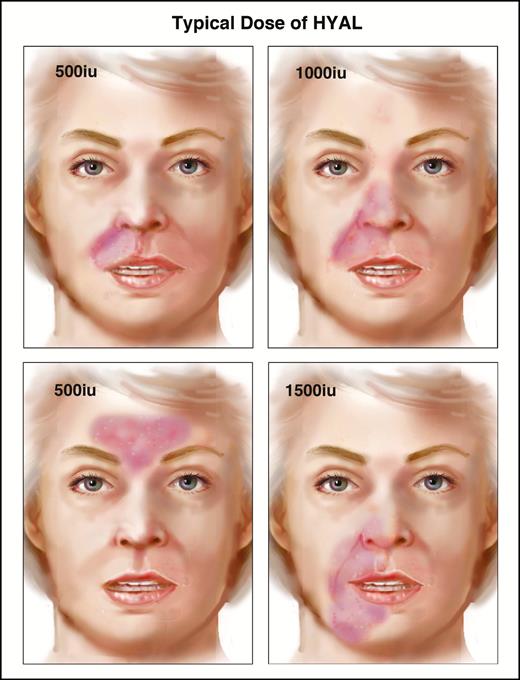
This suggested to the author that perhaps we were not using enough HYAL in at least some of these patients (otherwise, why did some patients heal completely, while others had crusting and scars, dyschromia, etc?). This lead to the “HYAL flooding hypothesis” (further discussed below), namely that HA filler obstructed arteries needed to be bathed in high concentrations of HYAL for long enough periods of time to dissolve the filler. This concept is illustrated in Figure 2, showing that the recommended dosage of HYAL increases stepwise with the numbers of anatomical areas involved. If more parts of the face are involved with ischemia, then larger doses of HYAL will be needed to treat that individual. We use ischemic surface area as a proxy for tissue volume (not the volume of intravascular filler − the amount of filler within the intravascular space is essentially irrelevant, and not clinically observable at the bedside). We can assume that all the arteries in the ischemic areas of the face are completely filled with HA filler. In order to obtain a sufficient concentration of HYAL (ie, mg/cc3) a larger volume of tissue (the denominator) means that a larger amount of HYAL is required.
Some common patterns of injury, and the typical doses used by the author for each. HYAL is injected into the ischemic tissues and gentle massage is then used to maximize the contact time between the ischemic tissues and HYAL. The tissues may be edematous, but gentle massage following injection of HYAL will tend to decrease this. Massage is important to help maximize contact between hyaluronidase and the filler causing the obstruction.
Analysis of Hyaluronidase Protocol
The newest hyaluronidase protocol for the treatment of filler embolism involves several important changes. First came the realization that HYAL was the critical factor in treatment. None of the ancillary treatments previously recommended (topical nitropaste, hyperbaric oxygen, etc.) are used at all any more, except for one baby aspirin per day for seven days (ASA is given to reduce platelet activity, but direct evidence for its benefit is lacking in this situation). The author has not recommended or used any of the ancillary treatments for over 2 years now.
With experience came the second important observation that dosing should depend on the quantity of tissues adversely affected (ie, one dose does not fit all). This is based upon the assumption that HYAL has to be used in quantities sufficient to bathe the obstructed vessels in a concentration sufficient to hydrolyse the filler causing the obstruction. A vessel that can no longer transport fresh blood because it is blocked by HA must be bathed (flooded) by a sufficient concentration of HYAL to diffuse across the arterial wall and then break down the HA to metabolic products small enough to pass through the capillary system. We cannot possibly know where the obstructed vessels are within the block of ischemic tissue. We must assume that all the vessels within the zone of ischemia are obstructed, and treatment must involve flooding the entire zone with sufficient HYAL concentration to promote complete hydrolysis of the HA filler (which, again, could be anywhere within the ischemic zone’s arterial system). With this view, partial breakdown of HA is insufficient, because partial breakdown products can still obstruct blood flow (although they may be pushed further downstream by arterial pressure). Thus when we are advising “HYAL flooding” what we mean to say is that we want to ensure that the obstructed vessels are soaking in a sufficient concentration of the enzyme to promote complete hydrolysis of the HA within the vessels. We assume that partial hydrolysis is insufficient. The author also suggests that partial breakdown may result in further obstruction downstream.
In fact, in this view, partial breakdown may result in new areas of ischemia (or at least, a zone of injury of a different shape than at presentation, as is sometimes seen), as these breakdown products may be carried downstream past areas where collateral vessels are bypassing the initial obstruction. In fact, this is the mechanism by which we posit that the phenomenon of “delayed onset” ischemia occurs, when dilution or blood pressure moves an embolus further downstream. A vessel containing an embolus of filler might not be yet causing ischemia, since collaterals may initially be completely by-passing the obstruction. As the filler makes its way downstream, it may then block the collaterals and then manifest the ischemia.
Clinical assessment of the patient must be ongoing and persistent. The goal of treatment is complete dissolution of the offending filler obstruction. To accomplish this, there must be sufficient concentration of HYAL in the right location long enough to result in (sufficiently) complete hydrolysis (Figure 3). The number of qualifications specified here are intentional, since a deficiency in any one of these elements is a recipe for failure to achieve the stated goal of complete resolution of the obstruction. In vitro, the response time for hydrolysis depends on the actual filler being tested. Let’s assume that a filler will take, roughly speaking, a few hours to break down, since the HYAL has to diffuse across the intact arterial wall and hydrolyse the filler. (Crosslinked fillers will take longer times to degrade with HYAL, whereas in contrast, in the author’s in vitro testing, non-crosslinked HA was hydrolyzed almost instantaneously, with complete liquefaction within just a few seconds of direct contact). It is known that HYAL itself is actively metabolized by the human body, so it is being deactivated at some rate as soon as it is injected. As swelling fluid accumulates from leaky capillaries in the ischemic environment, the HYAL is also being diluted. Finally, we know that as HYAL degrades the ground substance, it begins to diffuse away from the original injection site. Thus we can see that the amount of HYAL we originally injected into a region will be partially deactivated (by natural anti-HYAL agents), diluted by swelling fluids, and will physically diffuse away from the region where we want to maintain a high concentration. These three factors all act to reduce HYAL action, and the author suggests that these are reasons that we should act to top up the HYAL on a frequent basis to maintain the desired high concentration. The frequency of this topping up has to be determined in laboratory studies, but for now, the author has been using an hourly dosing schedule. This seems clinically to be safe and effective, but it may be overdoing it and dose ranging studies in a validated model are needed.
INTRA-ARTERIAL VERSUS EXTERNALLY APPLIED HYALURONIDASE
High volume vascular embolic events involve large bolus injections into the arterial system, and these typically require more vigorous treatment with Hyaluronidase because the zone of ischemia is larger, and dissolving the embolus takes longer. There is also the question regarding intra-arterial injection of HYAL, as opposed to the current strategy of simply bathing the external surface of the obstructed vessels with HYAL. With an arterial obstruction, as when clot busting drugs are used in intra-arterial treatments, there is the problem of maintaining the concentration of the drug where it is most needed. If the obstruction is complete, there is no flow past or through the obstruction, thus injecting HYAL into an occluded artery causes the HYAL to flow proximal to the obstruction. HYAL does work on the obstruction, but only at the site where it is exposed in the lumen of the vessel. In contrast, when flooding the entire zone of obstruction, the HYAL seems to break down the HA filler all along the vessel pathway, affecting the entire embolus at once. This hypothesis has not been tested however, and it remains to be proven whether external application of HYAL to the outside of a vessel is more or less effective than intra-arterial application of HYAL. Certainly for ease of use, external application of HYAL is very simple and appears to be safe and effective treatment, but there have been sporadic case reports at international meetings of treatment failures reversed with intra-arterial application of HYAL. This involves either a cut down procedure (to cannulate an artery) or super-selective angiography procedures that are not typically available except in tertiary care centers. For the time being, until evidence shows otherwise, external application by simply injecting HYAL into the affected areas and flooding the affected vessels through indirect diffusion appears to be a safe and effective solution in most cases. Clinical experience over that past few years has shown that the use of indirect soft tissue injection of HYAL alone without any ancillary treatments (ie, no nitropaste or hyperbaric oxygen etc.) has provided excellent results, superior to the previous protocol1 recommended.
TIME OF ONSET
Embolism probably most often happens contemporaneously with filler treatment, except in rare cases. There are some cases that report no evidence of problems at the end of treatment, but obvious signs of ischemia a few hours later. The author believes delay of onset of ischemia is rare, and at least some cases are likely due to perceptual blindness − ie, the injector simply is concentrating on the goals of symmetry, aesthetics, balance, and so on, and missing the signs of vascular ischemia.2 On occasion though, the author has heard of cases where the practitioner is adamant that the patient was completely fine at the end of treatment (ie, when the physician was actively assessing the patient for ischemia), only to discover rather obvious ischemia a few hours later. In these uncommon situations, the author hypothesizes that filler may be trapped in a vessel, perhaps at a bifurcation or branch point, with NO signs of distal ischemia because collateral vessels bypass the obstruction. At some point, this globule of filler may become dislodged by whatever mechanism, and then pass downstream to the precapillary arterioles, thus causing tissue ischemia. Despite the obvious violation of Occam’s Razor (where the simplest solution is usually correct), this two step process may turn out to have some validity in rare cases, but once again this is purely conjecture and the author has absolutely no hard evidence. In the typical event, the simple truth appears to be that the material seems to flow downstream within the arteries until it is constrained by size and can pass no further. It then simply blocks one or more arterial blood vessels, recalling that vessels can back fill towards a branch point by retrograde flow, and the filler can thus also obstruct nearby branches or even be passed towards a more distal zone through a main branch of a vessel. The zone of ischemia may be large or small sized depending on the amount of intravascular filler injected, and the relative importance of the specific blood vessel to the tissues in question. One could think of the vascular system as analogous to an Interstate highway system, where all points are connected, and it is quite possible to reach a distant exit ramp from any particular on ramp.
MECHANISM OF INJECTION:
HOW DOES FILLER GET INTO THE ARTERY?
It may be that most of these events are a result of direct intra-arterial injection, with the opening of the needle or cannula directly within the lumen of the vessel. Clearly, aspiration before injection might provide good feedback if bright red blood is seen, but a negative result may be misleading.3 In areas of scarred tissues, as from previous trauma, or even from a long series of former filler treatments, some special considerations may come into play. As a needle or cannula is passed into the tissues, this may create an artificial pathway for the filler to flow. In fact, this is a technique used called pre-tunneling, where a needle or cannula is passed through the tissues, and the filler is slowly injected upon withdrawal. If the tissues are scarred, then the needle track acts as a conduit for filler to flow through (Figure 5). A possible mechanism for embolism becomes apparent. If a vessel is penetrated by a needle or cannula, even if the filler is deposited at some distance beyond the vessel, the filler may back track along the needle pathway and then enter directly into the vessel, following a pathway of least resistance.
How penetration of a vessel might cause intravascular embolism, even though the tip of the cannula or needle is far past the lumen of the vessel. This is assumed to be more likely to occur when there is scar tissue present in the soft tissues. Penetration of scar tissues with a needle or cannula creates a pathway for filler to flow. The author hypothesizes that this type of problem may be become more prevalent with long term patients, since each filler treatment causes a small amount of scarring in the soft tissues.
Hyaluronidase Protocol Concclusions
There has been a significant improvement in the qualitative nature of the results with this high dose hyaluronidase protocol. With the previous hyaluronidase protocol, some patients had blisters, mild to moderate epidermal slough, and often had at least some degree permanent mild dermal scarring, as well as long lasting dischromia (particularly in patients of color). We used to consider this kind of mild scarring as a successful treatment, since it did not involve the serious degrees of tissue loss and the protracted healing by secondary intention that we had formerly observed in the completely untreated cases (often involving 6 weeks or more of dressing changes and aftercare). The author suggests redefining what a successful treatment entails. With this new protocol, we define successful treatment as complete resolution of ischemia with no epidermal scabs, scarring, or any secondary changes whatsoever.
The evidence from laboratory studies is that these events are a result of filler embolism occurring at the time of filler treatment. The working hypothesis is that flooding the tissues with a sufficient concentration of HYAL results in dissolution of the obstruction. We cannot know from clinical observation alone how much filler is present within the arterial system, nor can we know the precise location of the obstruction. For all practical purposes, we can assume that all the vessels in the affected areas are completely filled with HA filler. In order to relieve the obstruction, we need to ensure that a sufficient concentration of HYAL is present around the affected vessels for a period of time sufficient to ensure that diffusion can occur across the vessel wall. Thus there are several constraints on this system: the concentration of HYAL (which is degraded by deactivation by naturally occurring anti-HYAL factors in the tissues, dilution by leaking serum, and diffusion away from the site of obstruction; see Figure 4), the length of time the concentration is above the minimum required, the diffusion of HYAL into the vascular lumen, the resistance of tissues to hypoxia (which varies by tissue type). Since we cannot know where the obstruction is, exactly, we should assume that all the arteries in an ischemic area are obstructed, and that we need to treat that entire volume of tissue with sufficient HYAL to relieve the obstruction. We use the appearance of ischemia on the skin surface as a proxy for the volume of tissue that we must treat with HYAL. This is a complex system, with several time dependant variables and rates of change. In order to simplify treatment until a better system comes along, the author has been using a set of simple rules to govern treatment. These are not rigid rules, but rather flexible guidelines to help make clinical decisions. In general, larger areas of ischemia will require larger doses of HYAL given more frequently. Specifically, larger volumes of tissue will require more HYAL than smaller volumes of tissue.
We present a rough rule of thumb, using the lip, nose, and forehead as dose multipliers, with the standard dose of about 500 iu per area (Figure 2). For a single region, we recommend starting with a dose of about 500 iu every hour or so, until the ischemia is resolved (until skin color has returned and capillary refill time has returned to normal). For two areas, 1000 iu, and 1500 iu for three areas. We recommend keeping the patient in the clinic for observation, and retreating every 60 to 90 minutes until normal skin color returns. Typically, most will resolve after about three or four treatment sessions, but rarely there have been up to 8 or 9 re-injections of HYAL. Occasionally, because of exhaustion of both the patient and the clinician, the patient has been sent home overnight to return for more treatment the following day. It is important to realize that although treatment is urgent, it is not an emergency, as the soft tissues are relatively resistant to ischemia. As long as treatment is completed within about 72 hours (about 3 days) of onset of ischemia, success is common. These high, repeated doses of HYAL have been used for approximately 2 years in several dozen cases with excellent results, defined as complete resolution to normal, with no scabbing or other long lasting secondary changes (apart from the normal injection site reactions expected due to repeated injections). This is a clinical guideline that is done at the patient bedside with no special equipment or diagnostic laboratory requirements. Diagnosis is entirely clinical, done in the typical fashion with history and physical examination through observation and capillary refill tests.

Leave a Reply